Volodymyr Bezverkhniy - Review. Benzene on the basis of the three-electron bond. Theory of three-electron bond in the four works with brief comments (review). 2016.
- Название:Review. Benzene on the basis of the three-electron bond. Theory of three-electron bond in the four works with brief comments (review). 2016.
- Автор:
- Жанр:
- Издательство:Литагент Ридеро
- Год:неизвестен
- ISBN:9785448369469
- Рейтинг:
- Избранное:Добавить в избранное
-
Отзывы:
-
Ваша оценка:
Volodymyr Bezverkhniy - Review. Benzene on the basis of the three-electron bond. Theory of three-electron bond in the four works with brief comments (review). 2016. краткое содержание
Review. Benzene on the basis of the three-electron bond. Theory of three-electron bond in the four works with brief comments (review). 2016. - читать онлайн бесплатно ознакомительный отрывок
Интервал:
Закладка:
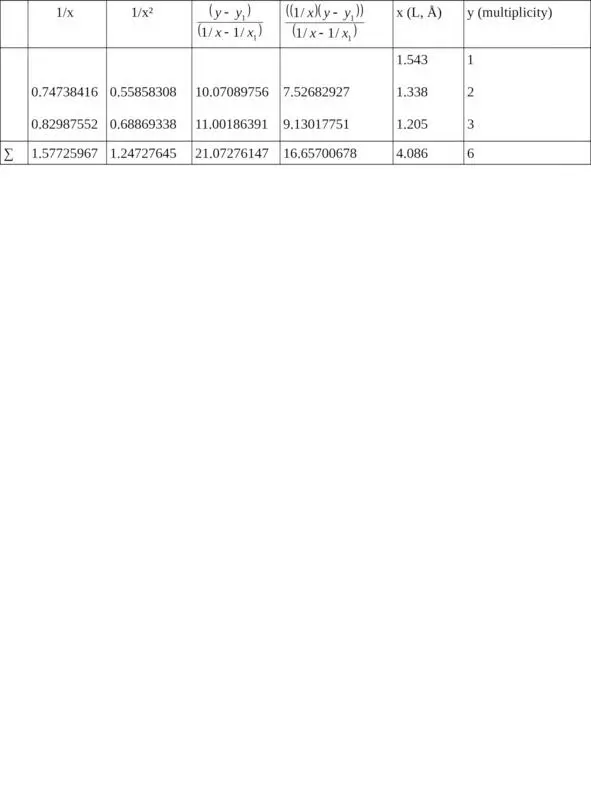
1/x 1= 0.64808814, x 1= 1.543, y 1= 1
Σ (1/x 2) = 1.66729469, Σ (1/x) = 2.22534781 when n = 3
c = 11.28562201,
b = – 5.67787529,
a = – 0.06040343
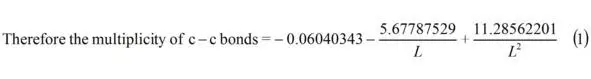
Let us find from the equation:
Multiplicity C—C (ethane) = 1.
Multiplicity C═C (ethylene) = 2.
Multiplicity C≡C (acetylene) = 3.
Multiplicity C—C (graphite) (L = 1.42 Å) = 1.538 ≈ 1.54.
Multiplicity C—C (benzene) (L = 1.397 Å) = 1.658.
As we can see the multiplicity C—C of benzene bond is 1.658 it is near the bond order of 1.667 calculated by the method MO [8, p. 48].
It should be noted that the а, b, с coefficients for this y = a + b/x + c/x² function in case of using three pairs of points (х 1, у 1), (х 2,у 2) and (х 3,у 3)are defined explicitly; actually, they (the coefficients) are assigned to these points. In that way we find these coefficients for working further with the equation. For making certain that this dependence y = a + b/x + c/x² describes well the Multiplicity = f (L) and E = f (L) functions it will take only to perform correlation for four or more points. For example, for the dependence Multiplicity = f (L) for C-C bonds we should add a fourth point (Lc—c = 1.397 Å, Multiplicity = 1.667) and obtain an equation with r² = 0.9923 and the coefficients а = – 0.55031721, b = – 4.31859233, с = 10.35465915.
As it is difficult, due to objective reason, to define four or more points for the Multiplicity = f (L) and E = f (L) equations for a separate bond type, we will find the а, b, с coefficients using three points (as a rule they are the data for single, double and triple bonds). The dependences obtained in such a way give good results as regards the bond multiplicity and energies.
We’ll find the dependence E = f (L) for the C—C bonds
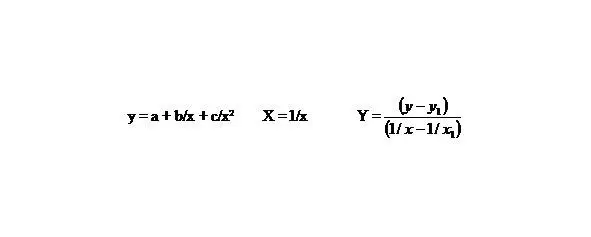
b 1= b + c/x 1,Y = b 1+ cX
As usual:
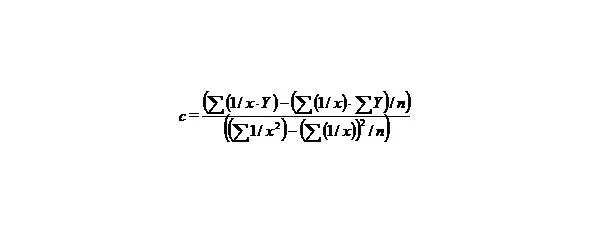
(7)
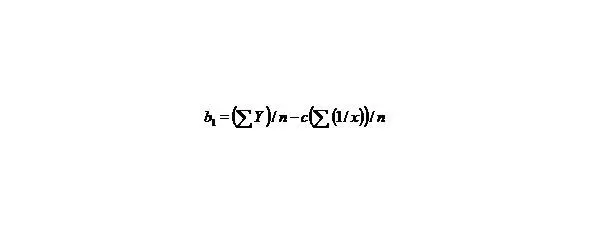
(8)
n—the number of given value Y.
Let us calculate a from the equation
∑y = na + b∑ (1/x) + c∑ (1/x 2), (9)
when n = 3.
Table 2. Calculation of ratios for relation E = f (L).
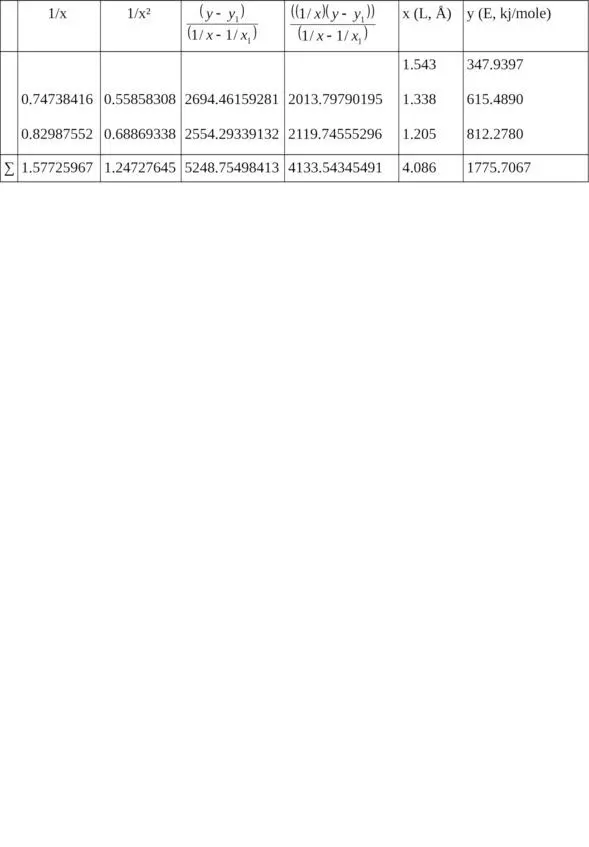
1/x 1= 0.64808814, x 1= 1.543, y 1= 347.9397
Σ (1/x 2) = 1.66729469, Σ (1/x) = 2.22534781 when n = 3
c = – 1699.18638789,
b = 5065.62912191,
a = – 2221.34518418

(2)
Let us calculate from the equation:
Ec—c (ethane) = 347.9397 kj/mole
Ec═c (ethylene) = 615.4890 kj/mole
Ec≡c (acetylene) = 812.2780 kj/mole.
2.3. Conclusion
As we can see, three-electron bond enables to explain aromaticity, find delocalization energy, understand aromatic bond’s specificity. Aromatic bond in benzene molecule is simultaneous interaction of three pairs of central electrons with opposite spins through the cycle. But whereas central electrons are the part of three-electron bond, then it is practically interaction of six three-electron bonds between themselves, that is expressed in three interactions through cycle plus six three-electron bonds. We shouldn’t forget in this system about important role of six atom nucleuses, around which aromatic system is formed. Properties of nucleuses especially their charge will influence on properties of aromatic system.
Finally, postulates of the three-electron bond theory (TBT) can be presented:
1) A chemical bond between two atoms may be established by means of three electrons with oppositely oriented spins (↑↓↑).
A • • • A (↑↓↑)
A • • • B (↑↓↑)
2) The electron shell of each atom in the stable molecule, ion, radical should have such a number of electrons which corresponds to the octet. A deviation from the octet results in an instability of a particle.
3) The state of the three-electron bond is determined by the octet rule.
4) The number of electrons participating in the chemical bond should be maximal and it’s then that the energy of the system will be minimal. Taking into consideration para 5 and 2.
5) In the course of establishing of the chemical bond electrons (their spins) are located in such a way that enables the interaction (attraction) to be maximal.
6) The aromatic bond is a three-electron bond in flat cyclic systems with a specific interaction of electrons through the cycle.
It is easy to show, that using three-electron bond one can explain paramagnetization and structure of oxygen molecule, structure of carboxylate anion, ozone, naphthalene and other organic and non-organic compounds. Let’s bring for the example structures of some compounds in terms of three-electron bond.
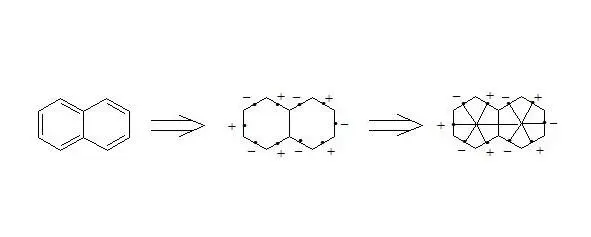
Naphthalene
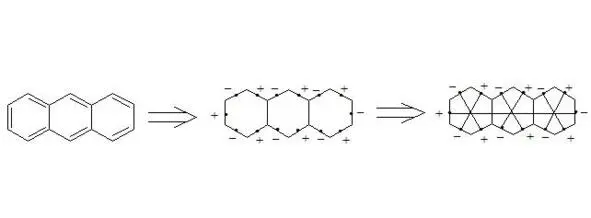
Anthracene
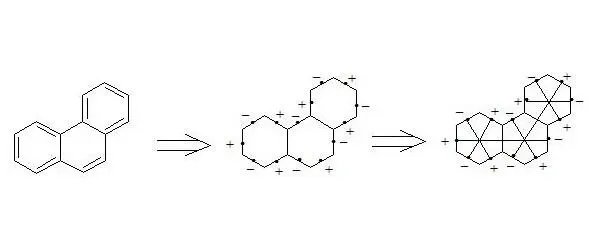
Phenanthrene
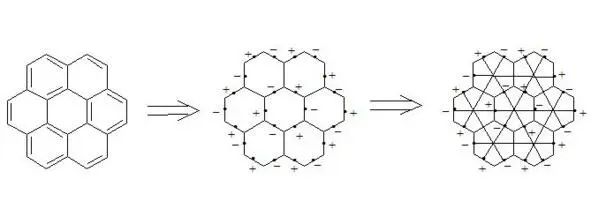
Coronene
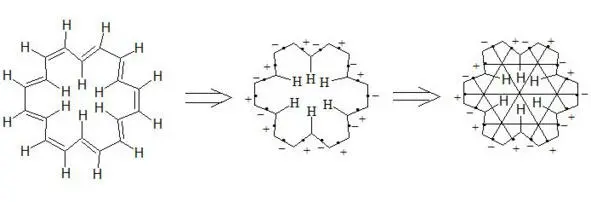
[18] -Annulene
It is interesting to note extreme symmetry of structures of naphthalene, anthracene, coronene and [18] -annulene, that is typical for the majority of aromatic compounds in general.
By the example of [18] -annulene it is possible to illustrate interaction through the cycle of central electrons of three-electron bonds. Interacting through the cycle, it shifts to the centre in the direction of inner atoms of hydrogen thus increasing electron density within the cycle and decreasing outside the cycle. And that’s why outside protons (12 Н) will give signals in the area of weaker field (reduction of electron density), and inner (6 Н) will give signals in the area of stronger field (increase of electron density). Thus this is observed in reality [13]. It also should be noted that inner protons bracing central electrons strengthen interaction through the cycle, and so stabilize aromatic system. But interaction through the cycle is decisive.
If aromatic system does not have inner protons, then outside protons will give signals in the area of weaker field (one of the features of aromatic compounds).
It is clear that in case of antiaromatic systems when there is no interaction (attraction) through the cycle, because central electrons have similar spins and push away, change in electron density in the centre of the cycle and outside the cycle will be reverse to aromatic systems.
Further we will continue demonstration of construction of organic and inorganic compounds.
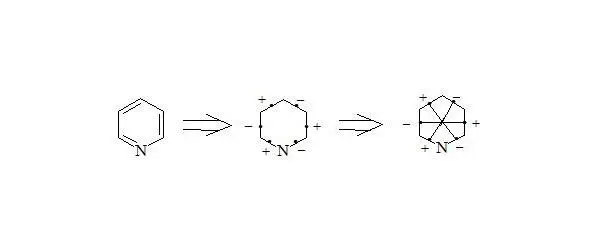
Pyridine
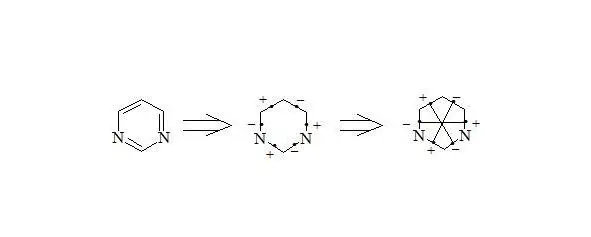
Pyrimidine
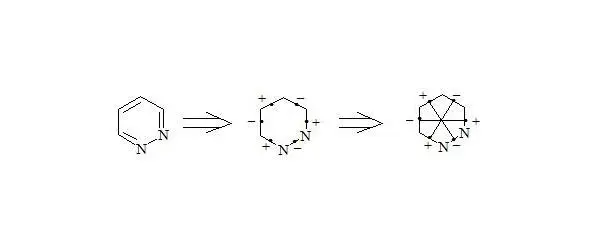
Pyridazine
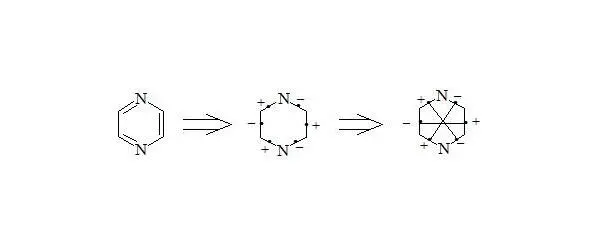
Pyrazine
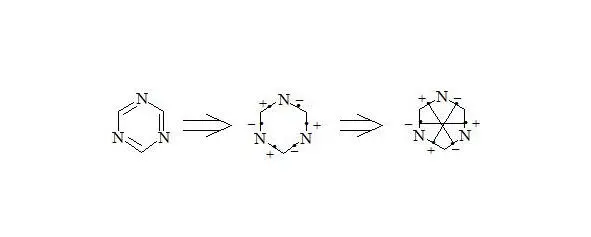
1,3,5-Triazine
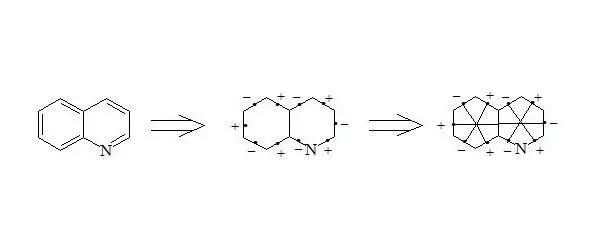
Quinoline
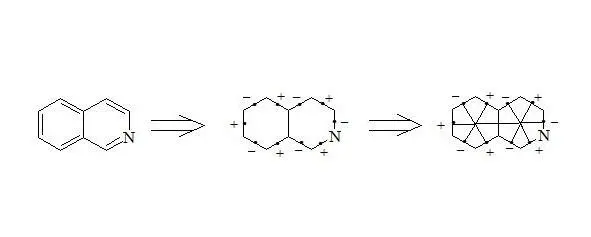
Isoquinoline
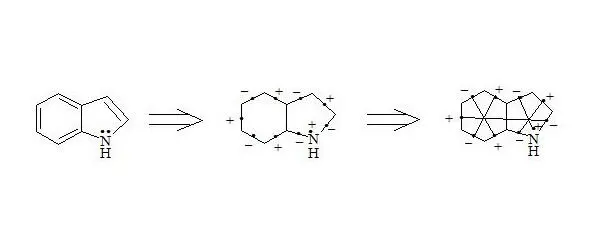
Indole
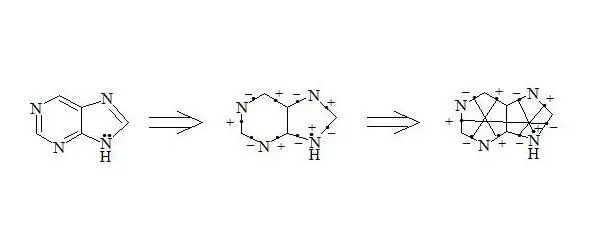
Purine
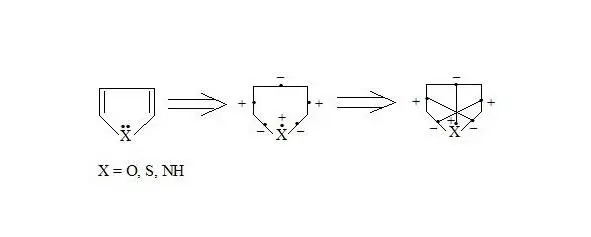
Furan, thiophene, pyrrole
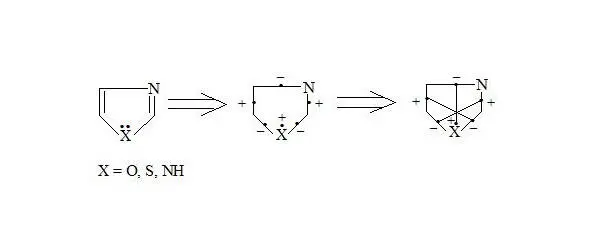
Oxazole, thiazole, imidazole
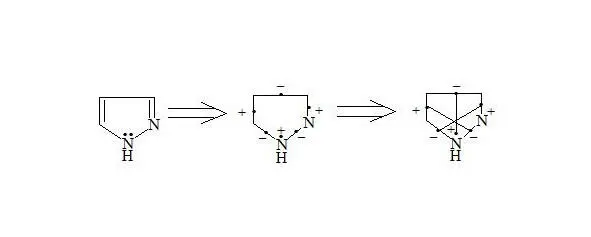
Pyrazole
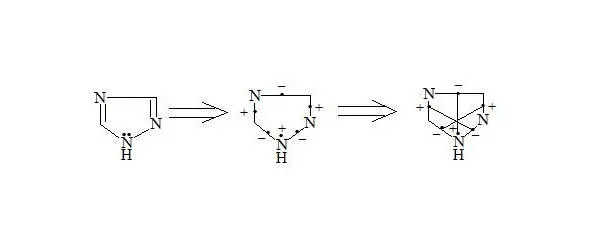
1,2,4-Triazole
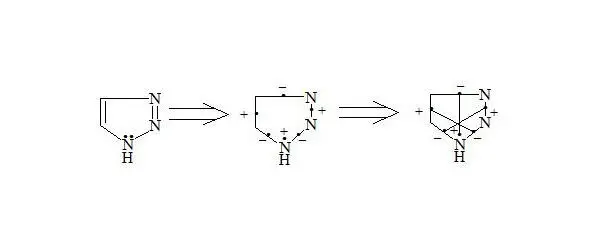
1H-1,2,3-Triazole
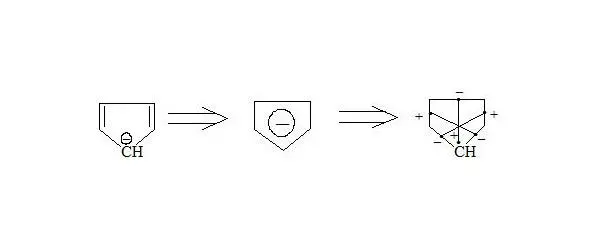
Cyclopentadienyle anion
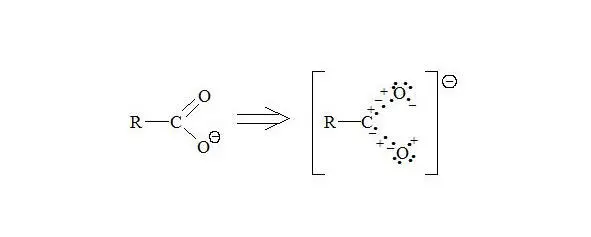
Carboxylate anion
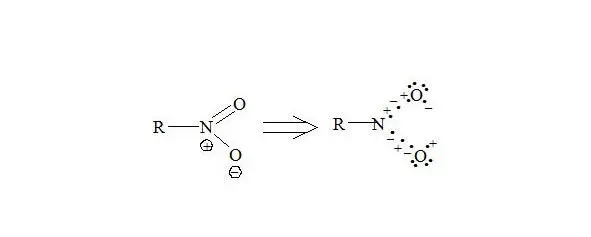
Nitro compounds
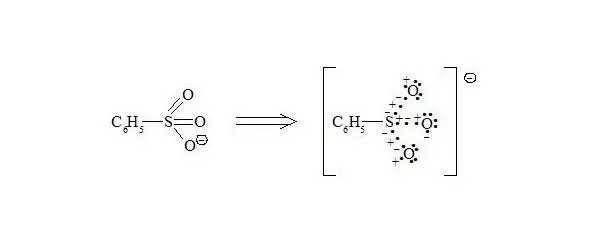
Sulfonate anion
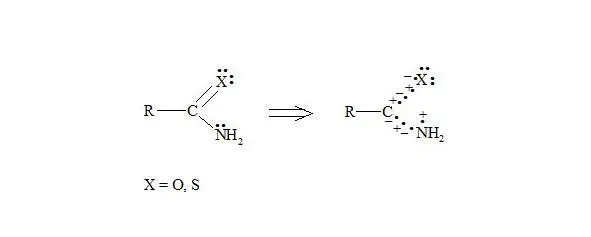
Organic acid amides and thioamides
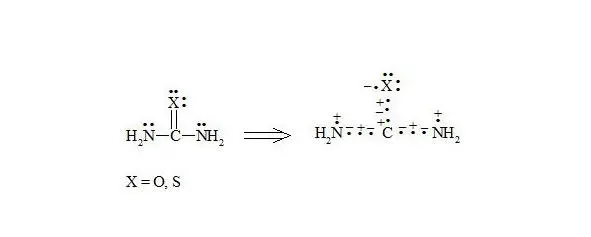
Urea and thiourea
Читать дальшеИнтервал:
Закладка:









