Я. Ившин - Metal Corrosion. Electroplating (Защита от металлов от коррозии. Гальванотехника)
- Название:Metal Corrosion. Electroplating (Защита от металлов от коррозии. Гальванотехника)
- Автор:
- Жанр:
- Издательство:Литагент БИБКОМ
- Год:2013
- Город:Казань
- ISBN:978-5-7882-1386-6
- Рейтинг:
- Избранное:Добавить в избранное
-
Отзывы:
-
Ваша оценка:
Я. Ившин - Metal Corrosion. Electroplating (Защита от металлов от коррозии. Гальванотехника) краткое содержание
Metal Corrosion. Electroplating (Защита от металлов от коррозии. Гальванотехника) - читать онлайн бесплатно ознакомительный отрывок
Интервал:
Закладка:
Я. Ившин, О. Лефтерова, Д. Рахимова
Metal Corrosion. Electroplating (Защита от металлов от коррозии. Гальванотехника)
Введение
Материалы пособия содержат общую информацию о коррозии металлов, ее типах и формах, включают аутентичные тексты, направленные на изучение способов и методов защиты металлов от коррозии, таких как гальваностегия, цинкование, никелирование, анодирование алюминием, хромирование, омеднение.
Данное пособие позволяет рассмотреть электрохимические процессы нанесения металлических, неметаллических и композиционных покрытий, методы исследования их структуры и функциональных свойств, сочетание электрохимических процессов с физическими методами обработки поверхности материалов.
Пособие состоит из 15 разделов, каждый из которых содержит комплекс из 17 заданий по работе с терминологией, устному и письменному переводу, устному изложению содержания текстов на русском и английском языках. Тексты способствуют формированию навыков использования научной и специальной терминологии в процессе письменного и устного общения в профессиональной сфере. Послетекстовые упражнения направлены на понимание прочитанного, развитие навыков устной речи и аннотирования.
Данное учебное пособие предназначено для студентов, магистрантов и аспирантов направления 250300 «Технология электрохимических производств» по специализации «Функциональная гальванотехника» и «Коррозия и защита металлов».
Unit 1
WHAT IS CORROSION?
1 . Read the international words, guess their meanings and give the Russian equivalents.
Corrosion, phenomenon, electrode, electrolyte, filtrate, laboratory, result, structure, radiator, acid, contribution, molecule, element, degradation, component.
2 . Read and translate the following verbs.
To contribute, to corrode, to protect, to destroy, to apply, to use, to affect, to observe, to establish, to make, to generate, to concentrate, to develop, to refer to, to replace, to perforate.
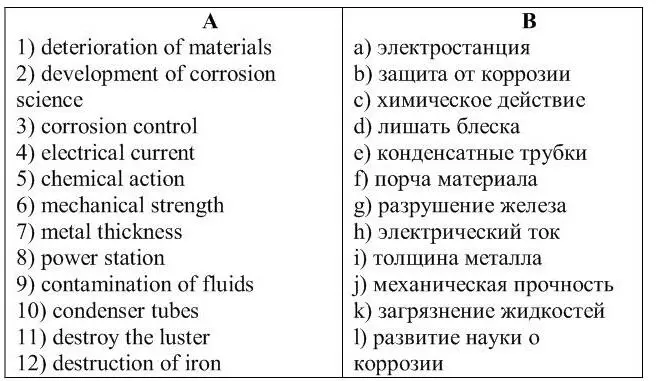
3 . Match the English phrases from column A with the Russian phrases in column B.
4 . Read and translate the text. Summarize it in Russian.
Corrosion is the deterioration of materials by chemical interaction with their environment. The term corrosion is sometimes also applied to the degradation of plastics, concrete and wood, but generally refers to metals. The most widely used metal is iron (usually as steel) which usually corrodes.
The word corrosion is as old as the earth but it has been known by different names. Corrosion is known commonly as rust, an undesirable phenomena which destroys the luster and beauty of objects and shortens their life. A Roman philosopher, Pliny (AD 23-79) wrote about the destruction of iron in his essay “Ferrum Corrumpitar”. Corrosion since ancient times has affected not only the quality of daily lives of people, but also their technical progress.
Philosophers, writers and scientists observed corrosion and mentioned it in their writings: Pliny the elder, Herodotus, Lomonosov, Austin, Thenard, Hall, Davy and De la Rive.
The most important contributions were later made by Faraday who established a quantitative relationship between chemical action and electric current. Ideas on corrosion control started to be generated at the beginning of nineteenth century. Whitney provided a scientific basis for corrosion control based on electrochemical observation. As early as in eighteenth century it was observed that iron corrodes rapidly in dilute nitric acid but remains unattacked in concentrated nitric acid. Considerable progress towards the modern understanding of corrosion was made by the contributions of Evans, Uhlig and Fontana. Corrosion laboratories established in M.I.T., USA and University of Cambridge, UK, contributed significantly to the growth and development of corrosion science and technology as a multi disciplinary subject. In recent years, corrosion science and engineering has become an integral part of engineering education globally. (From Trakia Journal of Sciences, vol.3, № 7, 2005) .
5 . Read the text again. Decide whether the following statements are true or false.
1. Corrosion is the degradation of materials due to interactions with their environments.
2. A French philosopher, Pliny (AD 23-79) wrote about the iron compounds in his essay “Ferrum Corrumpitar”.
3. Faraday established a qualitative relationship between oxidising action and electric current.
4. Whitney provided a scientific basis for corrosion control based on the cathodic protection.
5. In recent years, corrosion science and engineering has disappeared in engineering education.
6 . Read the text. What is its main idea?
Корро́зия (от лат. corrosio – разъедание) – это самопроизвольное разрушение металлов в результате химического или физико-химического взаимодействия с окружающей средой. В общем случае это разрушение любого материала, будь то металл или керамика, дерево или полимер. Причиной коррозии служит термодинамическая неустойчивость конструкционных материалов к воздействию веществ, находящихся в контактирующей с ними среде.
Коррозия металлов – разрушение металлов вследствие химического или электрохимического взаимодействия их с коррозионной средой. Наиболее часто при коррозии металл окисляется с образованием ионов металла, которые при дальнейших превращениях дают различные продукты коррозии. Коррозия может быть вызвана как химическим, так и электрохимическим процессом. Соответственно различают химическую и электрохимическую коррозию металлов. (From forexaw.com) .
7. Work in pairs. Translate the following text from English into Russian.
The major harmful effects of corrosion can be summarized as follows:
1. Reduction of metal thickness leading to loss of mechanical strength and structural failure or breakdown.
2. Hazards or injuries to people arising from structural failure or breakdown (e.g. bridges, cars, aircraft).
3. Loss of time in availability of profile-making industrial equipment.
4. Reduced value of goods due to deterioration of appearance.
5. Contamination of fluids in vessels and pipes.
6. Perforation of vessels and pipes allowing escape of their contents and possible harm to the surroundings. For example a leaky domestic radiator can cause expensive damage to carpets and decorations, while corrosive sea water may enter the boilers of a power station if the condenser tubes perforate.
7. Loss of technically important surface properties of a metallic component. These could include frictional and bearing properties, ease of fluid flow over a pipe surface, electrical conductivity of contacts, surface reflectivity or heat transfer across a surface.
8. Mechanical damage to valves, pumps, etc, or blockage of pipes by solid corrosion products.
8 . Work in pairs. Interpret the following passage sentence by sentence.
Corrosion is a natural and costly process of destruction like earthquakes, tornados, floods and volcanic eruptions, with one major difference. Сorrosion can be prevented or at least controlled. Several definitions of corrosion are reproduced below:
(A) Corrosion is the surface wastage that occurs when metals are exposed to reactive environments.
(B) Corrosion is the result of interaction between a metal and environments which results in its gradual destruction.
(C) Corrosion is an aspect of the decay of materials by chemical or biological agents.
(D) Corrosion is an extractive metallurgy in reverse.
(E) Corrosion is the deterioration of materials as a result of reaction with its environment (Fontana).
(F) Corrosion is the destructive attack of a metal by chemical or electrochemical reaction with the environment (Uhlig).
Despite different definitions, it can be observed that corrosion is basically the result of interaction between materials and their environment.
9 . Give English equivalents to the following word combinations and learn them by heart:
химическое взаимодействие
физико-химическое взаимодействие
термодинамическая неустойчивость
разрушение металлов
металл окисляется
электрохимический процесс
коррозийная среда
причины коррозии
10 . Give Russian equivalents to the following word combinations and learn them by heart:
reduction of metal thickness
loss of mechanical strength
structural failure
perforation of vessels and pipes
profile-making industrial equipment
electrical conductivity
blockage of pipes
solid corrosion products
11 . Translate the following sentences into Russian.
1. Millions of dollars are lost each year due to the corrosion of iron and steel.
2. The problem with iron as well as many other metals is that the oxide formed by oxidation does not firmly adhere to the surface of the metal and flakes off easily causing "pitting".
3. Extensive pitting eventually causes structural weakness and disintegration of the metal.
4. Corrosion occurs in the presence of moisture.
5. The formation of rust can occur at some distance away from the actual pitting or erosion of iron.
Читать дальшеИнтервал:
Закладка:









